ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH

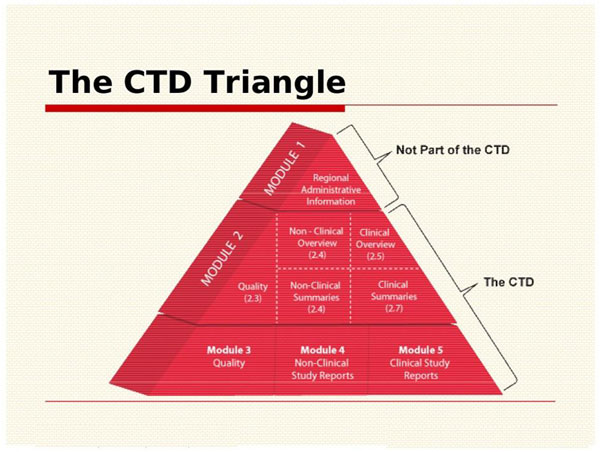
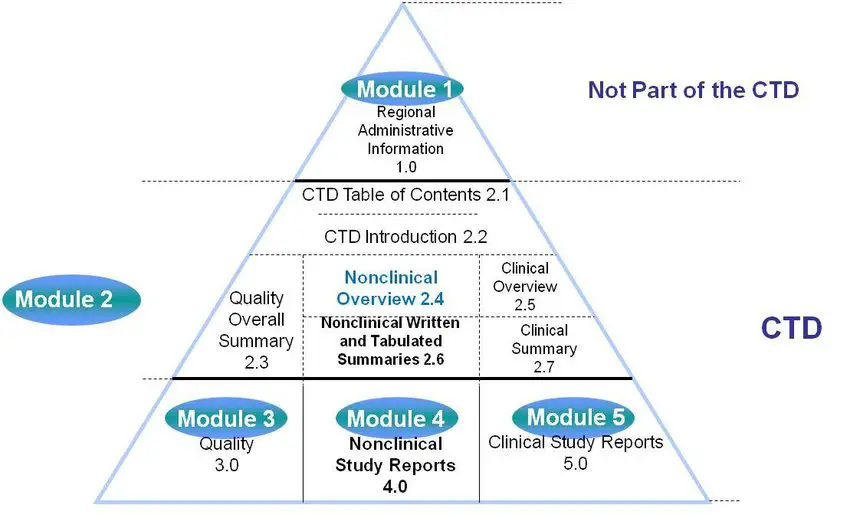
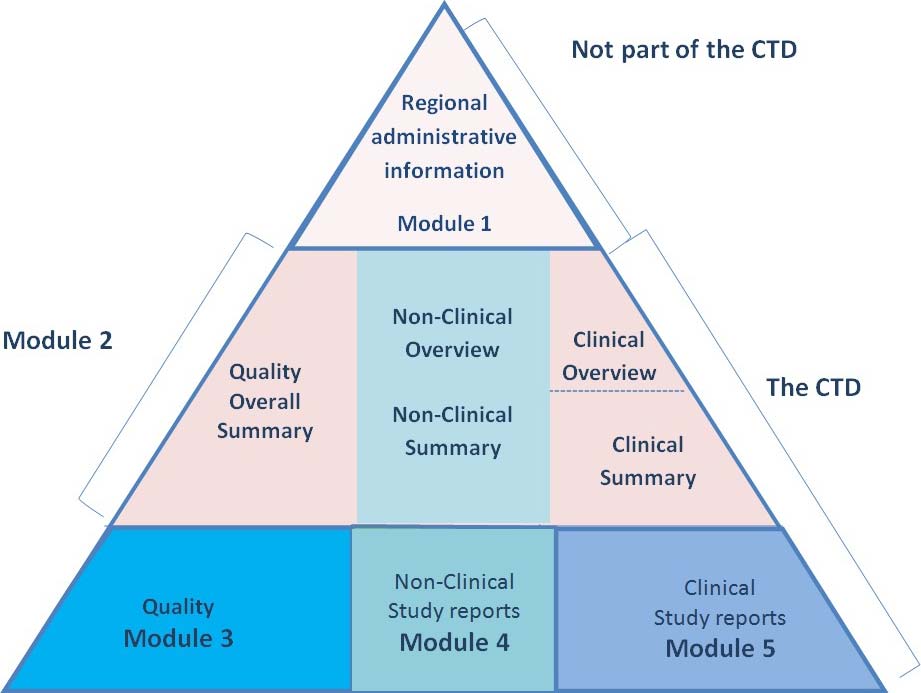
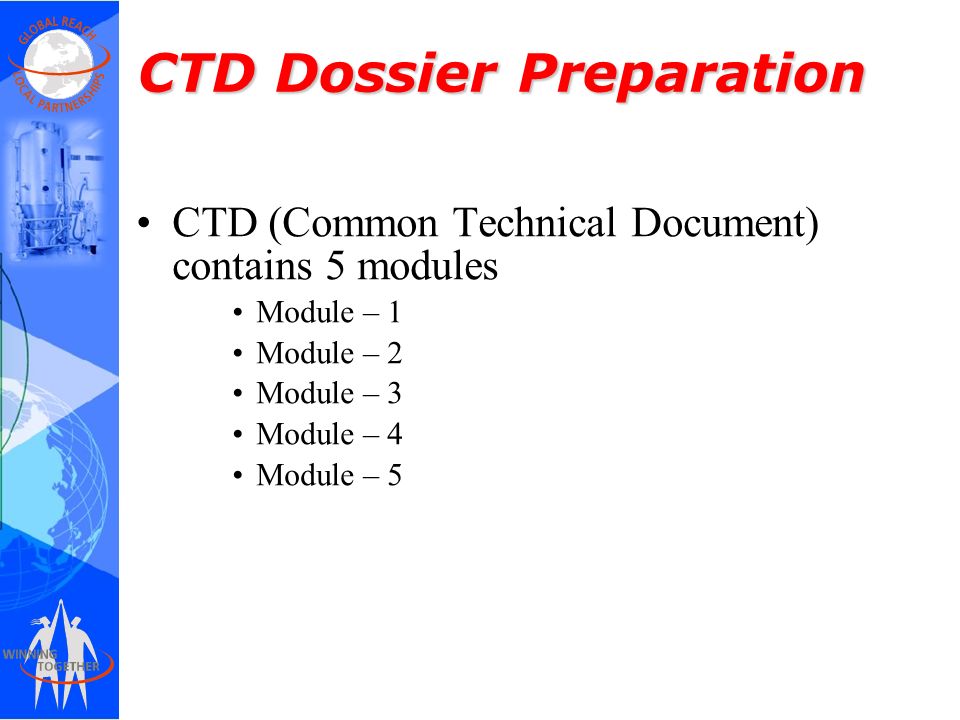
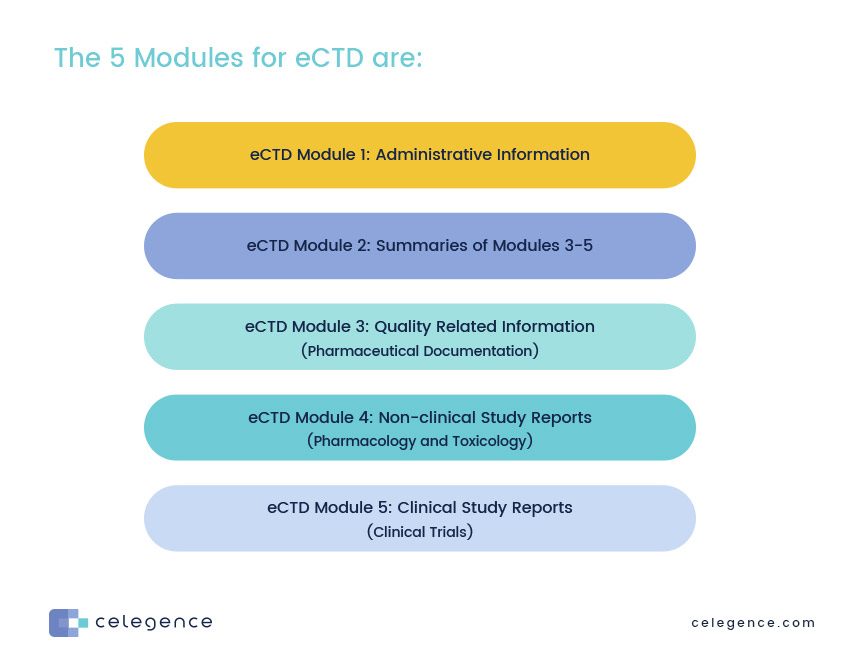
ClinProve - CTD Structure The Common Technical Document is divided into five modules: 1. Administrative and prescribing information 2. Overview and summary of modules 3 to 5 3. Quality (pharmaceutical documentation) 4.