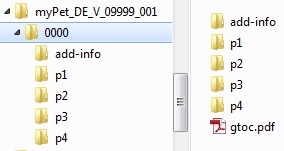
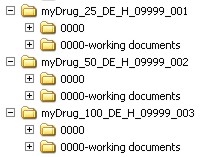
Horn Pharmaceutical Consulting - regulatory requirements, marketing authorisation, regulatory strategies, regulatory Authorities EMA, FDA, CTD , IMPD/IND, Variations and changes, orphan drug applications, SMEs ,Small and Medium-sized Enterprises
eCTD - Neue Wege der elektronischen Einreichung - Vernetzung elektronischer regulatorischer Prozesse -
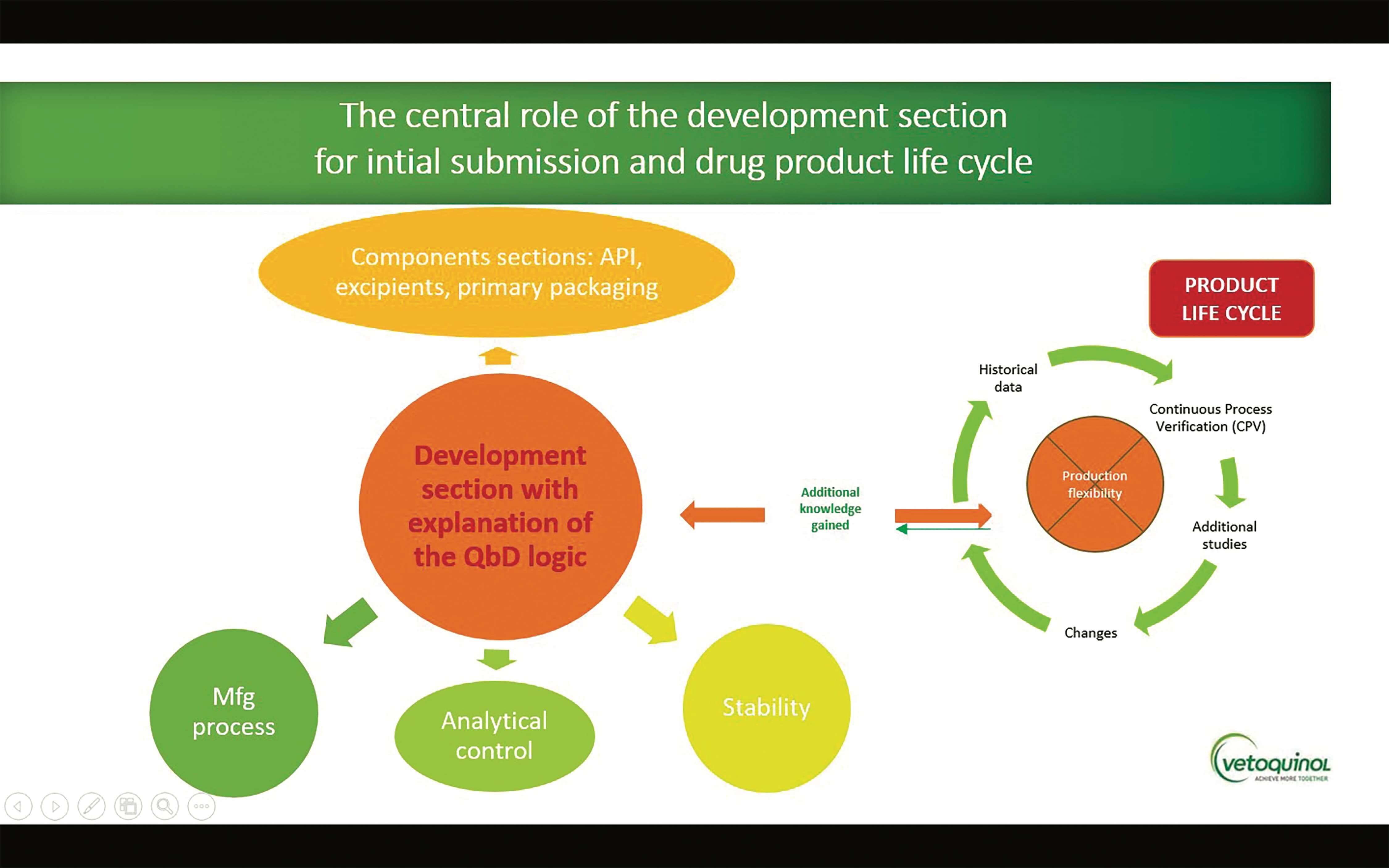
(1).png)