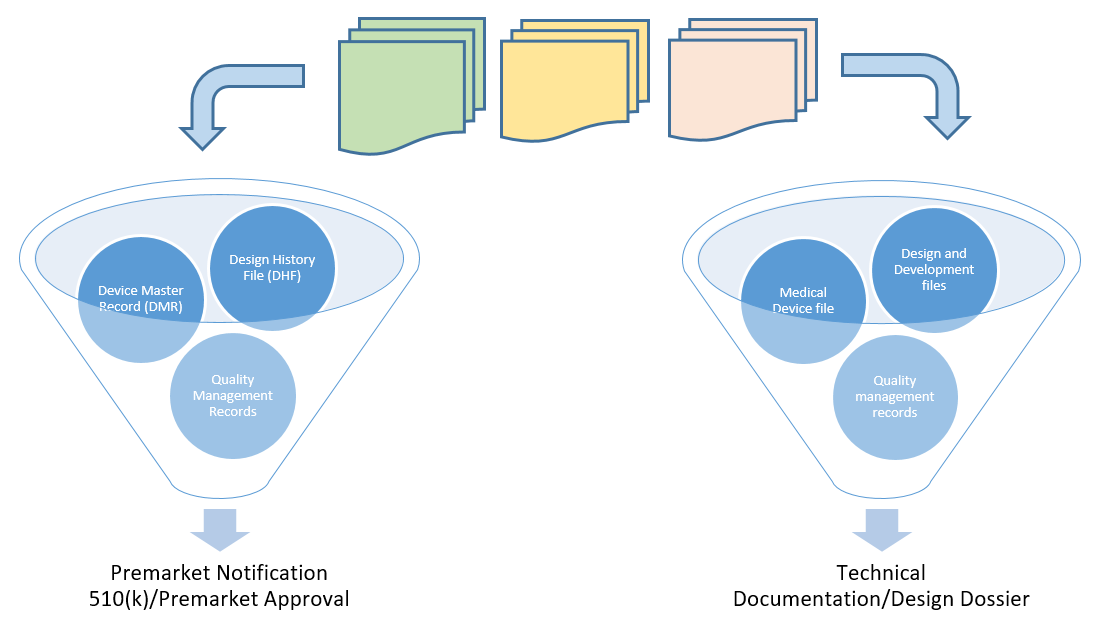
Design History Files (DHF), Device Master Records (DMR), Device History Records (DHR), Technical Documentation - The Requirements

Fillable Online Design History File (DHF) vs. Device Master Record (DMR) vs. Device ... Fax Email Print - pdfFiller

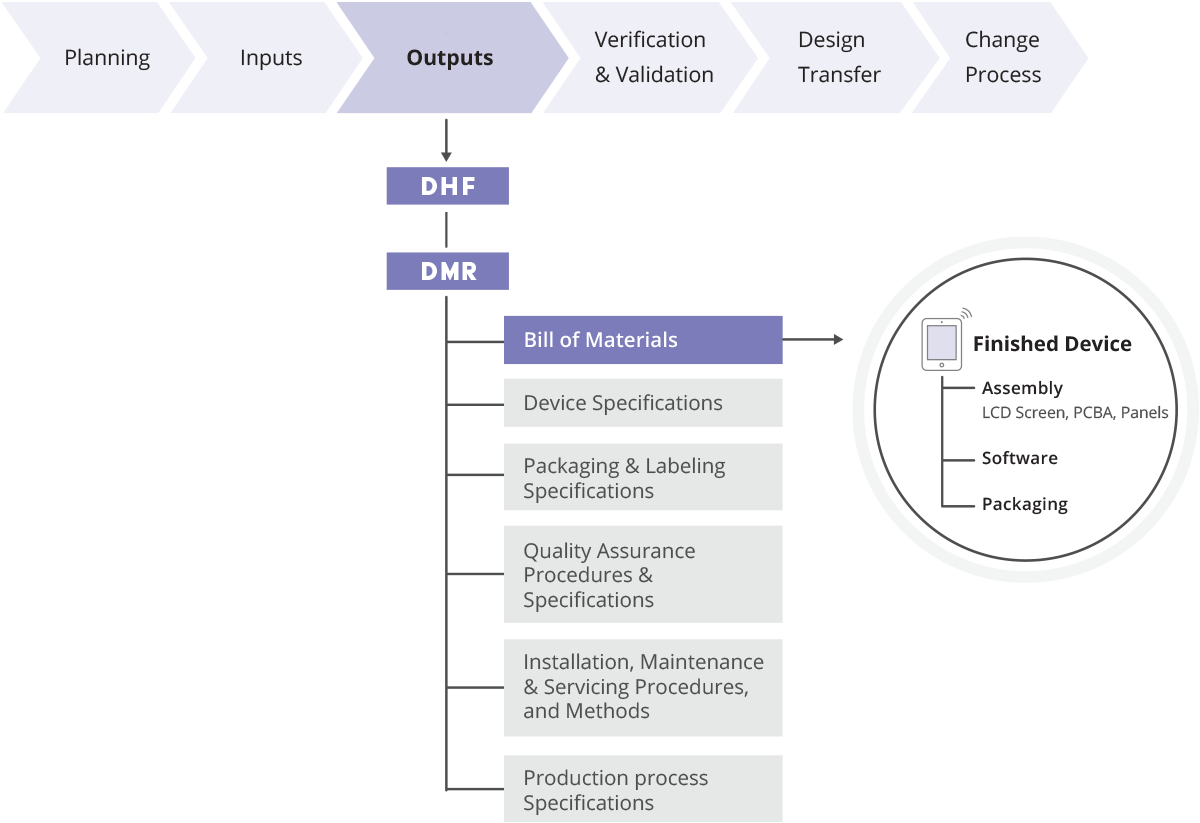
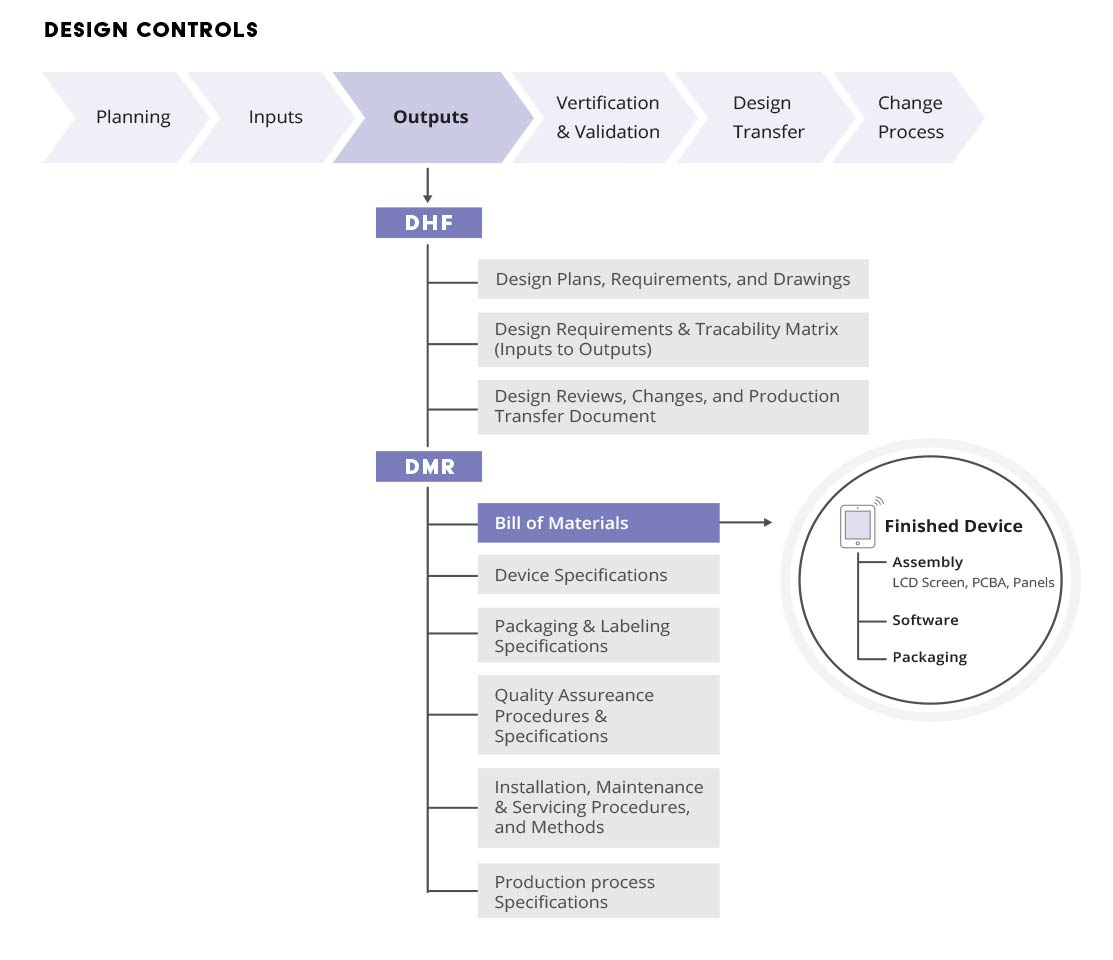
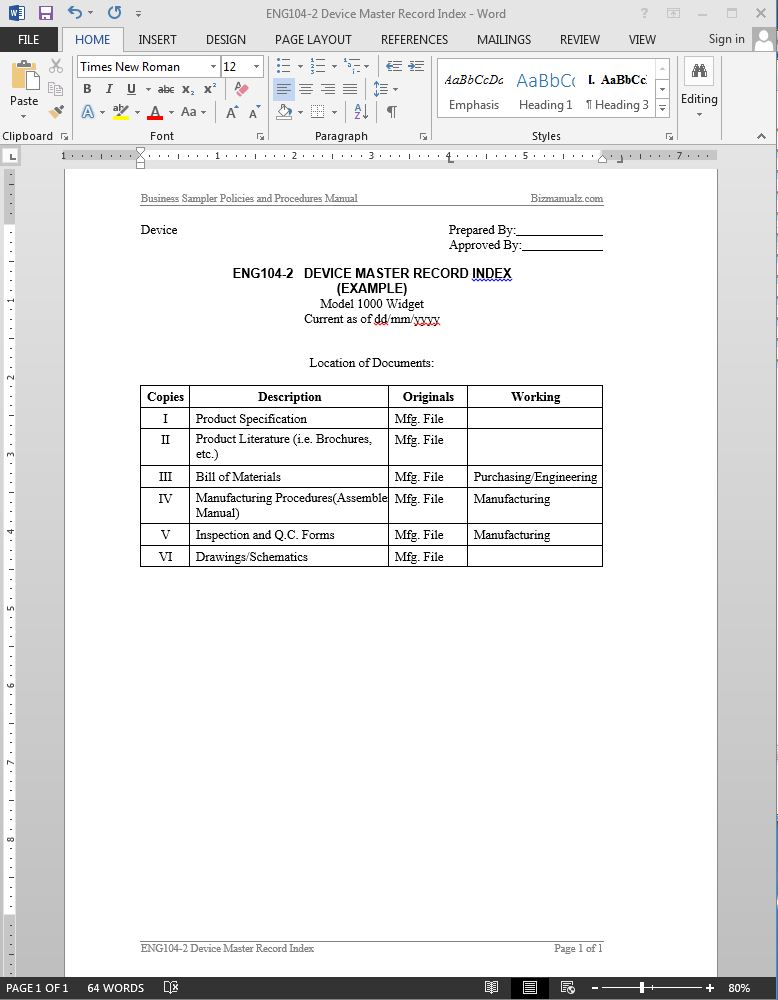
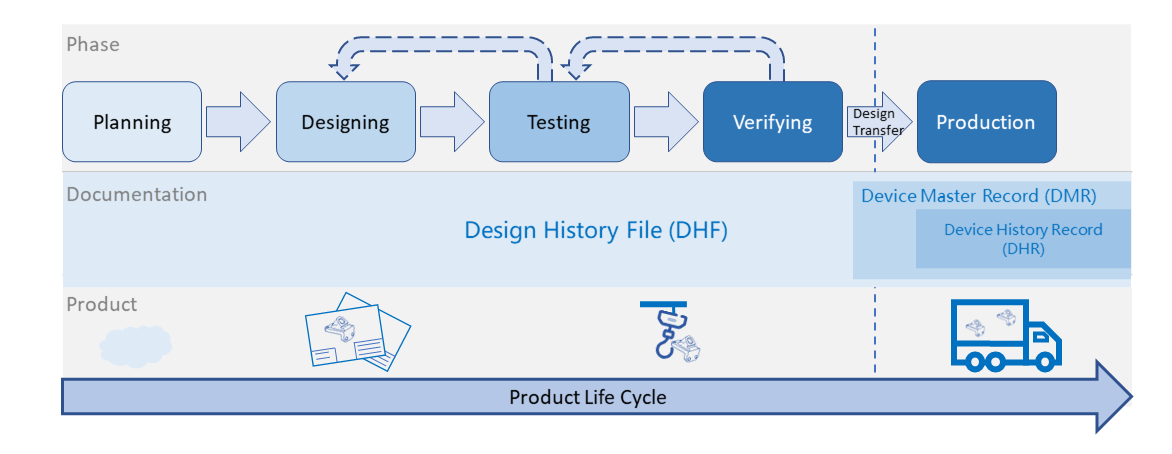
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR): What's the Difference?










-Tech%20File.png)


![Design History File 系列探討]:Design History File (DHF) 的定義以及在品質系統中所扮演的角色! Design History File 系列探討]:Design History File (DHF) 的定義以及在品質系統中所扮演的角色!](https://4.bp.blogspot.com/-jvxLiH6dyok/WxUDmoc__nI/AAAAAAAAC6k/G-88f2agk2kLLWvUoIbZzqqHIQ0leCBDwCLcBGAs/s1600/DHF%2B%2526%2BDMR%2B%2526%2BDHR.png)