A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S) | PharmaTutor

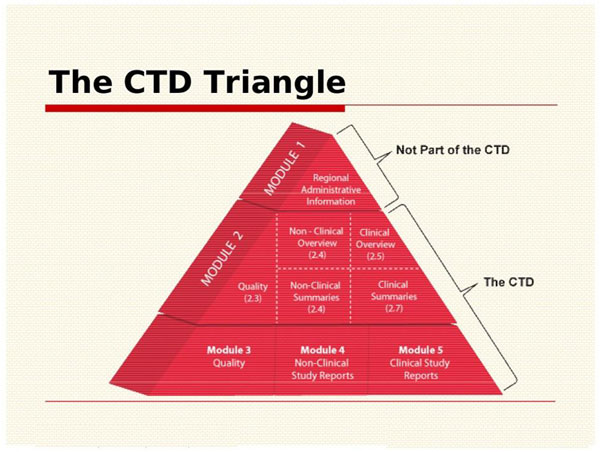
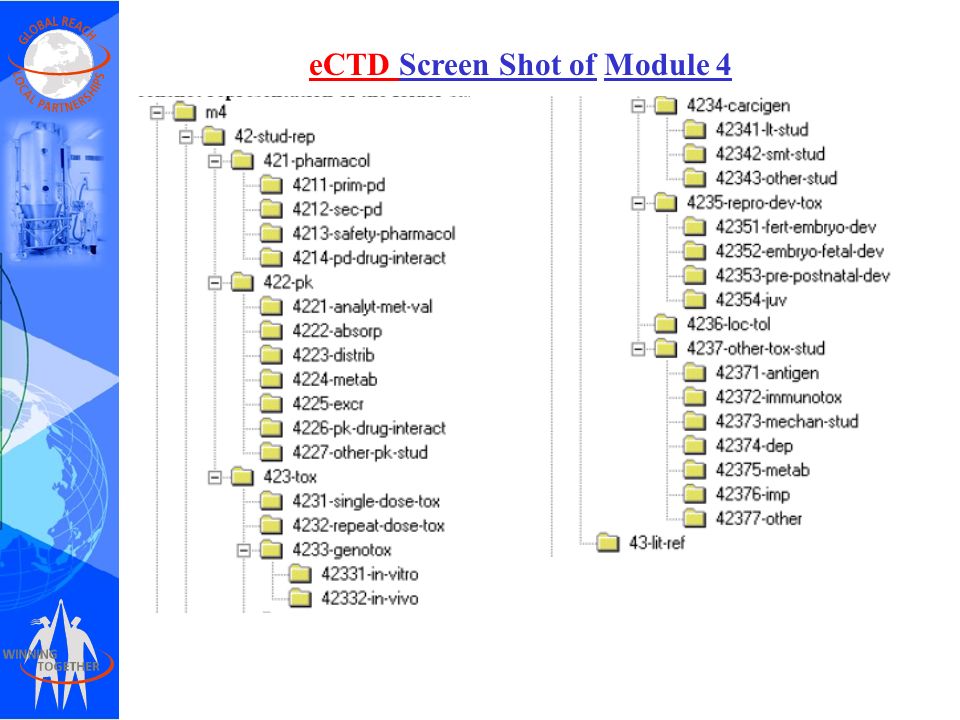
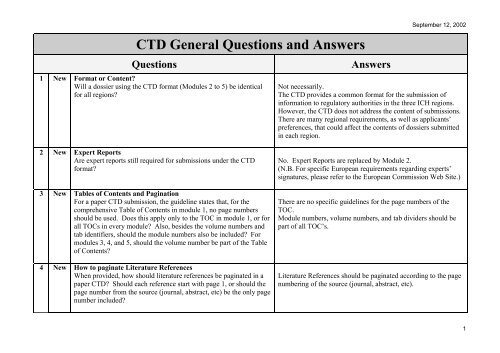
Last Update June 13 ' ToC of Module 1 or overall ToC, including Module ToC of the CTD (Mod 2,3,4,5) Module 1 Module 3Module 4Module ppt download

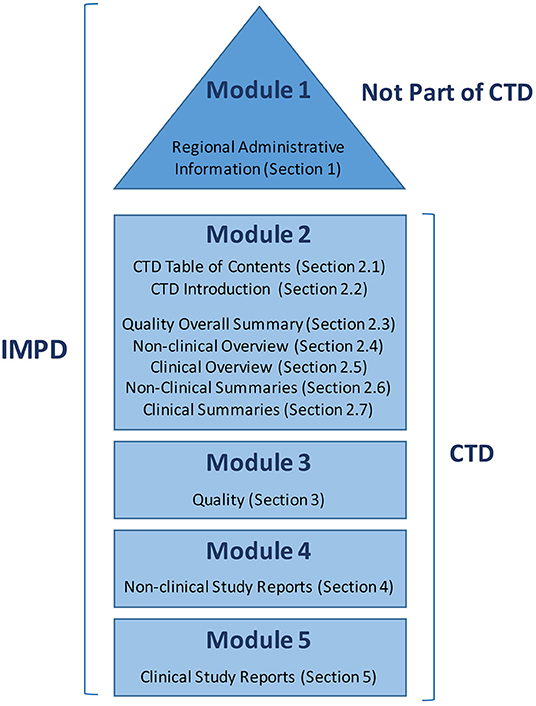
Representation of the components of the CTD. The nonclinical components... | Download Scientific Diagram
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience

Challenges for the registration of vaccines in emerging countries: Differences in dossier requirements, application and evaluation processes - ScienceDirect